Potassium Dihydrogen Phosphate: Uses & Safety Guide
Potassium Dihydrogen Phosphate: A Versatile Compound Powering Industries

- April 7, 2025
- By Akshita Patel
When you think of essential compounds in food, medicine, and industry, Potassium Dihydrogen Phosphate (KH₂PO₄) might not be the first thing that comes to mind. But this unassuming, water-soluble salt plays a crucial role in everything from stabilizing your favorite sports drinks to enhancing pharmaceutical formulations and supporting industrial processes.
Whether you’re a food scientist, a healthcare professional, or curious about the hidden chemistry behind everyday products, this compound has more applications than you’d expect. Let’s explore how KH₂PO₄ is shaping multiple industries and why it deserves more recognition!
What is Potassium Dihydrogen Phosphate?
Potassium Dihydrogen Phosphate (KH₂PO₄) is an inorganic compound that serves as a potassium and phosphate in various industries. It is widely used in food processing, pharmaceuticals, and laboratory research due to its stability, solubility, and buffering capabilities.
Molecular Formula and Structure
- Chemical formula: KH₂PO₄
- Composition: Contains potassium (K), hydrogen (H), phosphorus (P), and oxygen (O).
- Structure: Composed of a phosphate (PO₄) group bonded to hydrogen and potassium ions, making it a monopotassium phosphate compound.
Physical Properties
- Appearance: Colorless or white crystalline powder.
- Solubility: Highly water-soluble, making it ideal for liquid formulations.
- Melting Point: Decomposes upon heating beyond 252°C (486°F).
- Odor and Taste: Odorless with a slightly salty taste when used as a food additive.
Chemical Behavior
- Acidic Nature: KH₂PO₄ is a mildly acidic compound due to its hydrogen phosphate group.
- Buffering Role: It acts as an effective pH buffer, maintaining stability in food, pharmaceuticals, and laboratory solutions.
- Reaction with Bases: When mixed with strong bases, it can form dipotassium phosphate (K₂HPO₄), adjusting pH levels accordingly.
Due to its unique combination of solubility, stability, and buffering properties, Potassium Dihydrogen Phosphate is a key ingredient in a variety of scientific and industrial applications.
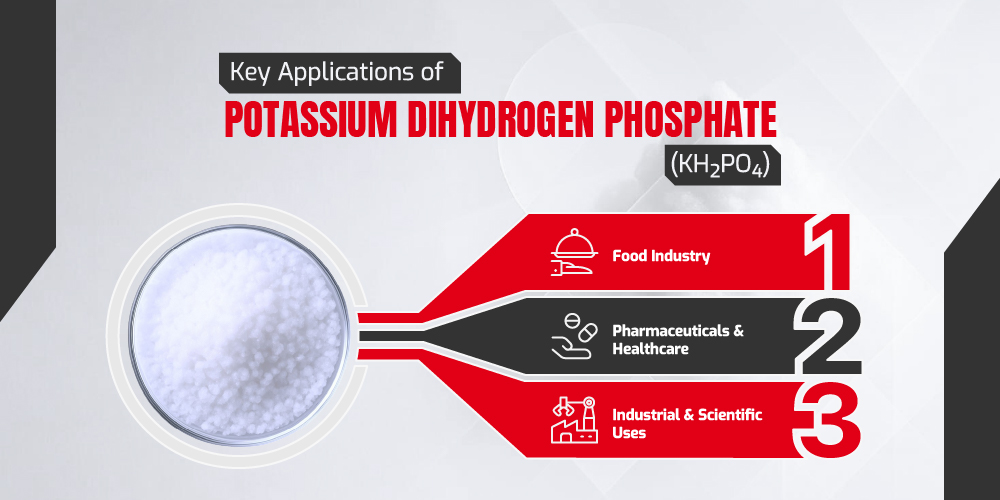
Key Applications of Potassium Dihydrogen Phosphate (KH₂PO₄)
Potassium Dihydrogen Phosphate is widely used across multiple industries due to its unique chemical properties. Its water solubility, buffering capacity, and pH regulation make it an essential component in food production, pharmaceuticals, and industrial applications. Let’s explore its key applications in detail.
Food Industry
- Food Additive (E340) in Processed Foods and Beverages: Potassium Dihydrogen Phosphate is approved as a food additive (E340) and is used in numerous food products for its ability to stabilize, emulsify, and regulate acidity. It is commonly found in:
- Dairy Products
- Used in cheese processing to improve texture and prevent syneresis (the separation of liquid from solid).
- Helps in powdered milk and creamers to prevent clumping and improve solubility.
- Enhances yogurt consistency by acting as a stabilizer.
- Baked Goods
- Works as a leavening agent in baking powders, ensuring proper dough rise.
- Strengthens gluten in bread and pastries, improving texture and elasticity.
- Prevents starch retrogradation in baked goods, keeping them fresh longer.
- Beverages & Sports Drinks
- Common in sports and electrolyte drinks for maintaining pH balance and hydration.
- Used in carbonated beverages to regulate acidity and improve shelf stability.
- Enhances cocoa-based drinks by improving dispersion and flavor stability.
- Dairy Products
- pH Regulation and Buffering Agent: Maintaining the right pH balance is crucial in food production to preserve freshness, prevent spoilage, and ensure taste consistency. KH₂PO₄ plays a vital role in:
- Acidifying soft drinks and fruit juices to enhance flavor stability.
- Neutralizing acidity in food formulations where excessive sourness needs to be controlled.
- Preventing microbial growth by maintaining an optimal pH for food safety.
- Emulsification and Anti-Caking Agent: Acts as an emulsifier in food formulations where water and oil need to be mixed, such as in:
- Salad dressings
- Processed meats
- Dairy-based sauces
- Works as an anti-caking agent in powdered food products like:
- Instant soups and sauces
- Powdered drink mixes
- Seasonings and spice blends
Pharmaceuticals & Healthcare
- Role in Medications and Dietary Supplements: Potassium Dihydrogen Phosphate is widely used in the pharmaceutical industry for its ability to provide essential nutrients and stabilize formulations. It is commonly found in:
- Electrolyte supplements for individuals with low potassium and phosphate levels.
- Phosphate-based medications are used to treat:
- Hypophosphatemia (low phosphate levels in the blood)
- Kidney stones (by increasing urinary phosphate levels)
- Osteoporosis (by improving bone mineralization)
Used as an excipient (inactive ingredient) in tablet formulations to ensure consistent dissolution and absorption.
- Intravenous (IV) Fluids and Electrolyte Balance: In hospitals and emergency care settings, KH₂PO₄ is used in intravenous (IV) therapy to:
- Replenish phosphorus levels in critically ill patients suffering from malnutrition or metabolic disorders.
- Treat electrolyte imbalances caused by dehydration, kidney disease, or severe diarrhea.
- Maintain acid-base balance in cases of diabetic ketoacidosis (DKA) and respiratory acidosis.
- Support cardiovascular health by ensuring proper nerve and muscle function.
- Application in Dental and Oral Care Products: Used in toothpaste and mouthwashes to maintain a stable pH and prevent plaque buildup.
- Helps in enamel remineralization, reducing tooth sensitivity.
- Prevents calcium phosphate precipitation, ensuring long-term oral health benefits.
Industrial & Scientific Uses
- Laboratory & Scientific Research: KH₂PO₄ is a key component in scientific research and laboratory experiments, primarily due to its ability to function as a pH buffer. It is used in:
- Biochemical and molecular biology research for stabilizing pH-sensitive reactions.
- Cell culture media provide essential nutrients for microbial and mammalian cell growth.
- Pharmaceutical and vaccine development, ensuring consistent formulation stability.
- Protein purification processes where maintaining an optimal pH is crucial.
- Fire Extinguishers & Corrosion Inhibitors:
- Used in dry chemical fire extinguishers (ABC-type) for effective suppression of fires involving combustible materials, flammable liquids, and electrical equipment.
- Works by disrupting the combustion process and forming a protective layer over burning surfaces.
- Added to boiler water treatment solutions to protect metal pipes from corrosion.
- Used in industrial cooling systems to prevent scale buildup and rust formation.
- Ceramics, Textile Processing, and Metal Treatment
- Used as a fluxing agent, helping reduce the melting point of ceramic materials.
- Improves the durability and strength of ceramic coatings and glazes.
- Enhances color fastness in fabric dyeing processes, ensuring vibrant and long-lasting colors.
- Acts as a pH stabilizer in textile bleaching and printing.
- Used in metal surface treatment to prevent oxidation and improve coating adhesion.
- Helps in electroplating solutions, providing a uniform finish on metals like zinc, copper, and aluminum.
Potassium Dihydrogen Phosphate is an essential compound that contributes significantly to food safety, medical advancements, and industrial efficiency. From stabilizing sports drinks to supporting life-saving IV therapy and preventing metal corrosion, its impact is far-reaching. Understanding its key applications highlights how this seemingly simple compound plays a vital role in multiple industries, ensuring product stability, efficiency, and safety in modern manufacturing and healthcare.
Safety, Storage, and Handling of Potassium Dihydrogen Phosphate (KH₂PO₄)
- Proper Storage: Potassium Dihydrogen Phosphate (KH₂PO₄) is highly water-soluble and can absorb moisture from the air. To maintain its stability and effectiveness:
- Store in a cool, dry place, away from direct sunlight.
- Keep containers tightly sealed to prevent moisture absorption.
- Use non-reactive storage materials, such as plastic or glass, to avoid contamination.
- Safety Precautions for Handling: While KH₂PO₄ is generally considered safe, improper handling may cause minor health issues:
- Avoid direct contact with skin and eyes, as it may cause mild irritation.
- Wear protective gloves and goggles when handling large quantities.
- Use in well-ventilated areas to prevent accidental inhalation of dust particles.
In case of skin or eye contact, rinse with plenty of water and seek medical attention if irritation persists.
- Environmentally Safe Disposal: KH₂PO₄ is non-toxic to the environment when disposed of properly:
- Dilute with water before disposal to minimize concentration.
- Follow local regulations for chemical waste disposal.
Avoid releasing large amounts into soil or water bodies, as it may contribute to nutrient imbalances in ecosystems.
By following these safety, storage, and disposal guidelines, users can ensure the safe and responsible handling of Potassium Dihydrogen Phosphate in various applications.
Potassium Dihydrogen Phosphate might not be a household name, but its impact spans across industries—from enhancing food quality and pharmaceutical formulations to ensuring smooth industrial operations. Its unique chemical properties, including pH regulation, buffering capacity, and high solubility, make it an indispensable ingredient in modern manufacturing and research.
Whether it’s keeping your sports drink balanced, stabilizing life-saving IV fluids, or preventing corrosion in industrial systems, KH₂PO₄ plays a vital role in our daily lives. However, like any chemical, proper handling, storage, and disposal are essential to ensure safety and environmental responsibility.
As industries continue to evolve, the versatility and effectiveness of KH₂PO₄ will ensure its continued relevance in food production, healthcare, and beyond. Understanding its applications and safe usage not only enhances its efficiency but also promotes responsible and sustainable practices in various fields.

Akshita Patel
As an advocate for sustainability, Akshita is committed to driving positive change within the chemical industry. She actively seeks out environmentally friendly solutions and promotes the adoption of sustainable practices. Akshita believes that a balance between economic growth and ecological responsibility is crucial for the industry's long-term success. She is dedicated to finding innovative ways to minimize environmental impact while maximizing efficiency and profitability.
Related Blogs

- October 30, 2024
- By Akshita Patel
The Versatile Uses of Zinc Sulphate:.
From the food you eat to the products you use daily, zinc plays an unsung yet.

- March 4, 2025
- By Akshita Patel
Everything You Need to Know About.
Did you know that a tiny yet powerful compound is crucial in medicine and skincare? Meet.