The Comprehensive Guide to Di Potassium Hydrogen Phosphate (K2HPO4) - Annexe Chem Pvt Ltd
The Comprehensive Guide to Di Potassium Hydrogen Phosphate (K2HPO4)

- July 10, 2024
- By Akshita Patel
Di Potassium Hydrogen Phosphate may not be familiar to you, but it is an essential part of our everyday existence. This humble substance, which is chemically known as K2HPO4, has numerous roles in the food processing and agricultural sectors. This white, salt-like material is also known as dipotassium phosphate or potassium phosphate dibasic. It is a silent workhorse. It contributes to the health and freshness of your food, keeps your garden flourishing, and keeps your sports drinks balanced.
What, though, makes K2HPO4 unique? Although it appears straightforward, it has several intriguing twists up its sleeve.
Molecular structure: K2HPO4 consists of two potassium ions (K+) paired with a hydrogen phosphate ion (HPO42-). Picture a phosphorus atom at the center, surrounded by four oxygen atoms, with one hydrogen attached. This structure gives it some unique properties.
Physical characteristics: In its usual form, K2HPO4 is a white, odorless powder or crystalline solid. It’s hygroscopic, meaning it tends to absorb moisture from the air – leave it out, and it might start to clump. One of its standout features is its high solubility in water, dissolving readily to form a clear solution.
pH and buffering capacity: When dissolved in water, it yields a pH of 8.5–9.5 solution that is somewhat alkaline. But its capacity to cushion is perhaps its greatest strength. Even when adding bases or acids to a solution, it can aid in keeping the pH steady. This makes it extremely helpful in applications where maintaining pH control is essential, such as maintaining the ideal conditions for chemical reactions in labs or balancing the pH of your body in sports drinks.
This combination of properties – stable structure, easy solubility, and excellent buffering capacity – makes K2HPO4 a versatile compound in various industries.
Diverse Applications of Di Potassium Hydrogen Phosphate
Di Potassium Hydrogen Phosphate (K2HPO4) is a versatile compound with a wide range of applications across various industries. Let’s dive into its most significant uses:
Agriculture
Fertilizer use: K2HPO4 is a key player in the world of fertilizers. It gives plants the two vital elements they need, phosphorous and potassium. Phosphorus is essential for energy transfer and root development, whereas potassium aids in controlling water uptake and disease resistance in plants. K2HPO4 is a common fast-acting fertilizer used by farmers and gardeners, particularly for crops that require an increase in phosphorus during the flowering and fruiting periods.
Soil pH adjustment: Many plants thrive in slightly acidic to neutral soil. K2HPO4 can help nudge soil pH in the right direction. When applied to acidic soils, it can help raise the pH, making the soil more hospitable for crops that prefer less acidity. This pH-adjusting ability makes K2HPO4 a valuable tool for soil management in agriculture.
Food Industry
As a food additive (E340): In the European Union, K2HPO4 goes by the E-number E340. It’s approved as a food additive and serves several purposes. You might find it listed on the ingredients of various processed foods, where it acts as a stabilizer, acidity regulator, or sequestrant (a substance that improves the quality and stability of food products).
Functions in food processing: K2HPO4 is a jack-of-all-trades in food processing. It can:
- Act as a buffer to control acidity in foods and beverages
- Serve as an emulsifier in processed cheese, helping to blend oils and water
- Function as a nutrient supplement, adding potassium and phosphorus to fortified foods
- Help retain moisture in processed meats, improving texture and shelf life
Industrial Uses
Water treatment: K2HPO4 is an excellent corrosion inhibitor in the field of water treatment. It helps to prevent rust and prolong the life of pipes and equipment by creating a protective film on metal surfaces. It can aid in the regulation of scale formation in industrial water systems and is utilized in certain water-softening procedures.
Flame retardants: Safety first! Being a part of several flame retardant formulations, K2HPO4 helps prevent fires. It can create a barrier that slows the spread of flames when exposed to heat. Because of this characteristic, it can be used to create flame-resistant materials for a range of uses.
Research and Laboratory Applications
In the lab, K2HPO4 is a trusted workhorse. Its buffering capacity makes it invaluable in creating and maintaining specific pH environments for experiments and reactions. It’s often used in:
- Biological Research: As a component in growth media for microorganisms and cell cultures
- Biochemistry: In buffer solutions for enzyme studies and protein purification
- Analytical Chemistry: As a standard for calibrating pH meters
- Environmental Testing: In solutions for soil and water analysis
Moreover, its role in phosphate buffer systems makes it crucial in many biochemical processes and assays.
From farm to lab, and factory to dining table, Di Potassium Hydrogen Phosphate proves its worth across a spectrum of applications. Its unique properties make it an indispensable tool in modern agriculture, food production, industry, and scientific research. Next time you enjoy a perfectly textured processed cheese or admire a thriving crop field, remember – K2HPO4 might just be working its magic behind the scenes.
Market Trends and Future Outlook Of Di Potassium Hydrogen Phosphate
The demand for K2HPO4 has seen steady growth in recent years, primarily fueled by its diverse applications. The agriculture sector remains a major consumer, with an increasing focus on precision farming and soil health management. In the food industry, the trend towards processed and convenience foods has kept demand robust.
Industrial use, particularly in water treatment, has also been on the rise. As aging infrastructure in many countries requires modernization, the need for effective corrosion inhibitors like K2HPO4 has increased.
The research and biotechnology sectors continue to be significant consumers, with the compound’s buffering properties making it indispensable in many laboratory applications.
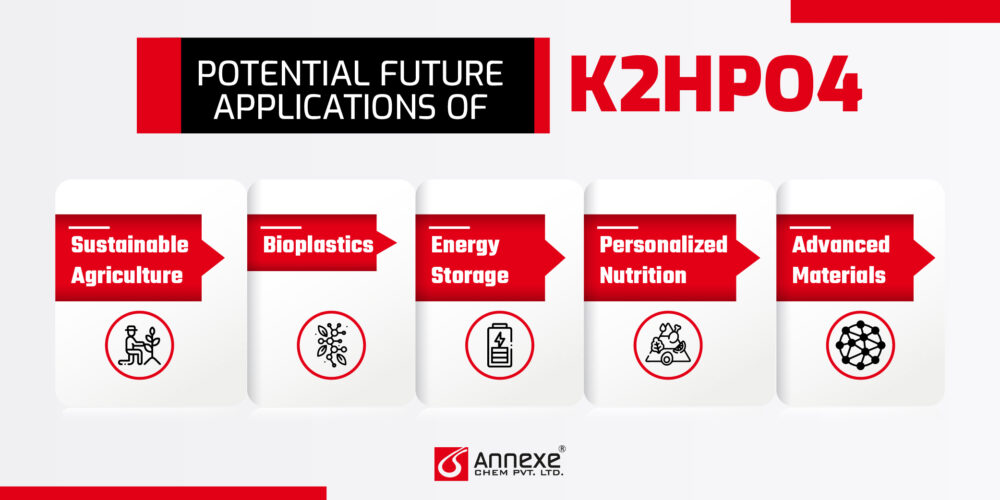
Potential future applications of K2HPO4:
- Sustainable Agriculture: As the world grapples with food security issues, K2HPO4’s role in enhancing crop yields could become even more critical. Its potential in hydroponic and vertical farming systems is particularly promising.
- Bioplastics: Research into biodegradable plastics has shown potential for phosphate-based compounds like K2HPO4 as additives to improve material properties.
- Energy Storage: Some studies suggest phosphate compounds could play a role in next-generation battery technologies, potentially opening a new market for K2HPO4.
- Personalized Nutrition: As the concept of tailored dietary supplements gains traction, K2HPO4’s use in fortified foods and beverages might expand.
- Advanced Materials: In nanotechnology and materials science, K2HPO4 could find new applications in creating specialized coatings or composites.
The future outlook for K2HPO4 appears promising, with its versatility likely to be its greatest asset. However, the market isn’t without challenges. Growing environmental concerns about phosphate runoff and eutrophication may lead to stricter regulations in some regions. This could drive innovation in application methods and potentially open up new markets for more environmentally friendly alternatives or precision application technologies.
Moreover, the push towards circular economy models could see increased interest in phosphate recovery and recycling processes, potentially affecting supply chains and pricing in the long term.
In conclusion, while Di Potassium Hydrogen Phosphate has been a quiet workhorse across industries for decades, its story is far from over. As we face new global challenges in food production, environmental protection, and technological advancement, this versatile compound is likely to continue playing a crucial role, adapting to new applications and market demands. The future of K2HPO4 promises to be as dynamic and multifaceted as the compound itself.

Akshita Patel
As an advocate for sustainability, Akshita is committed to driving positive change within the chemical industry. She actively seeks out environmentally friendly solutions and promotes the adoption of sustainable practices. Akshita believes that a balance between economic growth and ecological responsibility is crucial for the industry's long-term success. She is dedicated to finding innovative ways to minimize environmental impact while maximizing efficiency and profitability.