Calcium Acetate: Properties, Uses & Future Prospects | Annexe Chem
Calcium Acetate: A Comprehensive Guide to Its Properties, Uses, and Future Prospects

- May 7, 2024
- By Akshita Patel
Calcium acetate might not be a household name, but this versatile compound plays a crucial role in many industries and applications that impact our daily lives. Calcium acetate’s unique properties make it an indispensable ingredient in various processes, from the food we consume to the buildings we live and work in. This often-overlooked compound is a salt formed by the combination of calcium ions and acetate ions. As you delve deeper into the world of calcium acetate, you’ll uncover its fascinating history, production methods, and the cutting-edge research being conducted to expand its applications even further.
What is Calcium Acetate?
Calcium acetate is a calcium salt of acetic acid with the chemical formula Ca(C2H3O2)2. It is an ionic compound consisting of calcium cations (Ca2+) and acetate anions (C2H3O2-). The acetate anion is derived from acetic acid, a weak organic acid found in vinegar.
The structure of calcium acetate is characterized by the ionic bonding between the positively charged calcium ions and the negatively charged acetate ions. These ions arrange themselves in a crystalline lattice, forming a solid compound at room temperature.
Physical and Chemical Properties of Calcium Acetate
Physically, calcium acetate exists as a white, hygroscopic (moisture-absorbing) crystalline solid or powder. It has a slightly bitter taste and is odorless. Calcium acetate is highly soluble in water and also soluble in certain organic solvents like ethanol and methanol.
Chemically, calcium acetate is classified as a salt and exhibits properties typical of ionic compounds. It is a moderately strong electrolyte, capable of dissociating into its constituent ions when dissolved in water. Calcium acetate has a relatively high melting point of around 280°C (536°F) and decomposes upon further heating, releasing acetic acid and calcium oxide.
One of the notable properties of calcium acetate is its ability to regulate acidity and buffer solutions. It can act as a weak base or a weak acid, depending on the pH of the environment, making it useful in various applications that require pH control.
Occurrence of Calcium Acetate
Calcium acetate occurs naturally in certain plant materials, such as wood, and can be found in trace amounts in some fruits and vegetables. However, its natural occurrence is relatively limited, and most calcium acetate used commercially is produced synthetically.
Synthetic calcium acetate is manufactured through various chemical processes. One common method involves the reaction between calcium oxide (quicklime) or calcium hydroxide (slaked lime) and acetic acid. This reaction produces calcium acetate and water as byproducts.
Another synthetic route involves the reaction between calcium carbonate (limestone or chalk) and acetic acid, which also yields calcium acetate and carbon dioxide as a byproduct.
Calcium acetate can also be produced as a byproduct in certain industrial processes, such as the production of cellulose acetate or the fermentation of certain organic materials.
Overall, calcium acetate is a versatile compound with unique properties that make it valuable in various industries and applications. Its ability to regulate acidity, buffer solutions, and its high solubility contribute to its widespread use in fields ranging from food and pharmaceuticals to construction and environmental remediation.
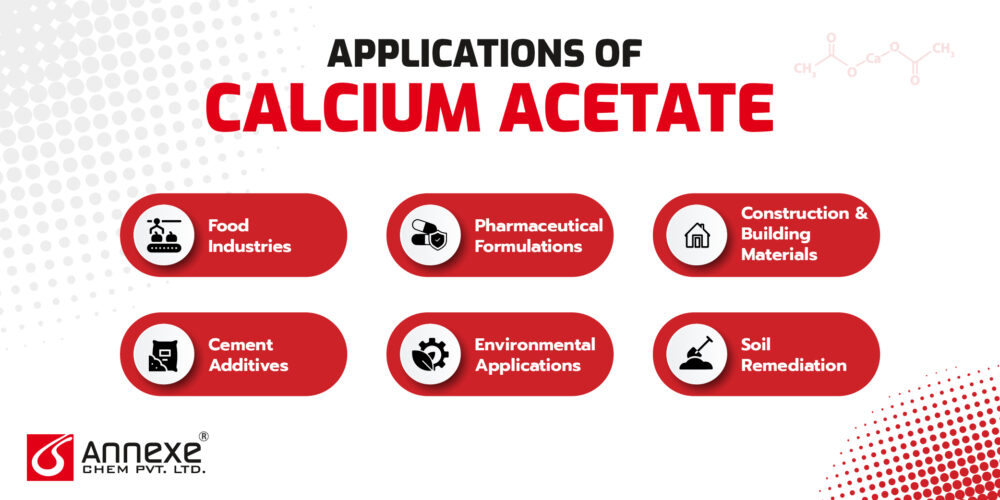
Applications of Calcium Acetate
- Food Industries
As a Food Additive (Preservative, Acidity Regulator), Calcium acetate plays a vital role in the food industry as a preservative and acidity regulator. Its ability to control pH levels and inhibit microbial growth makes it an effective food additive.
As a preservative, calcium acetate helps extend the shelf life of various food products by inhibiting the growth of bacteria, yeasts, and molds. It is particularly useful in preserving products with high moisture content, such as bakery items, processed meats, and certain dairy products.
Additionally, calcium acetate is used as an acidity regulator in foods. It can act as a buffer, helping to maintain the desired pH level in products like sauces, dressings, and beverages. This pH control is essential for ensuring product stability, preventing discoloration, and enhancing flavor profiles.
- In Pharmaceutical Formulations (Antacids, Calcium Supplements)
In the pharmaceutical industry, calcium acetate finds applications in the formulation of antacids and calcium supplements.
As an antacid, calcium acetate helps neutralize excess stomach acid, providing relief from conditions like heartburn, acid reflux, and indigestion. Its buffering capacity and ability to regulate pH make it an effective ingredient in over-the-counter and prescription antacid medications.
Calcium acetate is also used in the production of calcium supplements. These supplements are essential for maintaining strong bones, supporting muscle function, and preventing conditions like osteoporosis. Calcium acetate is often preferred over other calcium salts due to its high bioavailability and better absorption in the body.
- Construction and Building Materials
In the construction industry, calcium acetate is widely used as an admixture in concrete production. Admixtures are additives that enhance the properties and performance of concrete mixes.
When added to concrete, calcium acetate acts as an accelerator, reducing the setting time and allowing for faster construction schedules. It also improves the workability and pumpability of the concrete mixture, making it easier to handle and place.
Additionally, calcium acetate can enhance the durability and strength of concrete by improving its resistance to freezing and thawing cycles, as well as reducing the risk of corrosion in reinforced concrete structures.
- Cement Additives
Calcium acetate is also used as an additive in cement production. It can modify the setting time and workability of cement mixtures, making them more suitable for various construction applications.
By regulating the hydration rate of cement, calcium acetate helps control the initial and final setting times. This property is particularly useful in situations where quicker or slower setting times are desired, such as in cold or hot weather conditions, respectively.
Furthermore, calcium acetate can improve the overall performance of cement by enhancing its resistance to chemical attacks and reducing the risk of efflorescence.
- Environmental Applications
Calcium acetate finds applications in wastewater treatment processes, contributing to the removal of various contaminants and pollutants from water sources.
In wastewater treatment plants, calcium acetate is used as a coagulant and precipitant. It helps remove suspended solids, heavy metals, and other dissolved impurities by promoting their precipitation and facilitating their separation from the water.
Additionally, calcium acetate can be used in the treatment of industrial wastewater, where it aids in the removal of specific contaminants, such as sulfates and phosphates, ensuring that the treated water meets environmental regulations before being discharged or reused.
- Soil Remediation
Calcium acetate plays a role in soil remediation efforts, helping to address and mitigate contamination from various sources, including heavy metals, organic pollutants, and acidic soil conditions.
In soil remediation techniques, calcium acetate is used as a soil amendment or chelating agent. It can facilitate the immobilization of heavy metals, reducing their bioavailability and preventing them from entering the food chain or groundwater sources.
Furthermore, calcium acetate can help neutralize acidic soils, which can be detrimental to plant growth and ecosystem health. By buffering the soil pH, it creates a more favorable environment for microbial activity and plant development, supporting the restoration of contaminated or degraded land.
Calcium acetate’s versatility and unique properties make it an invaluable compound across various industries and applications. From enhancing food safety and supporting human health to improving construction materials and contributing to environmental remediation efforts, this unassuming salt continues to play a crucial role in our daily lives.
Emerging Applications and Trends
As scientists and researchers continue to explore the unique properties and potential of calcium acetate, new and innovative applications are emerging across various industries. The versatility of this compound has opened up possibilities for its use in cutting-edge technologies and novel applications.
One area of interest is the development of calcium acetate-based materials for biomedical applications. Researchers are investigating the use of calcium acetate in the fabrication of biodegradable and biocompatible materials for tissue engineering, drug delivery systems, and bioactive implants. The ability of calcium acetate to regulate pH and promote cell growth makes it a promising candidate for these applications.
Another emerging trend is the use of calcium acetate in agricultural practices. As the demand for sustainable and eco-friendly farming methods grows, calcium acetate is being explored as a potential soil amendment and plant growth promoter. Its ability to buffer soil pH and provide essential nutrients like calcium could contribute to improved crop yields and enhanced plant health.
Calcium acetate, a seemingly unassuming compound, has proven to be an indispensable ingredient in numerous industries and applications. From preserving our food and fortifying our health to enhancing construction materials and contributing to environmental remediation, this versatile salt has firmly established its significance in our daily lives. Looking ahead, the future prospects for calcium acetate are promising. Emerging applications in fields like biomedical engineering, sustainable agriculture, and energy storage highlight the compound’s potential to address global challenges and drive technological advancements. Ongoing research and development efforts are continuously expanding our understanding of calcium acetate, paving the way for new and innovative uses.
When it comes to acquiring high-quality calcium acetate for your specific needs, Annexe Chem Pvt Ltd stands as a trusted partner. With a commitment to excellence and a customer-centric approach, Annexe Chem Pvt Ltd ensures reliable supply and superior quality, making them the ideal choice for all your calcium acetate requirements.
Reach out to Annexe Chem Pvt Ltd to learn more about our calcium acetate solutions and how we can support your endeavors.

Akshita Patel
As an advocate for sustainability, Akshita is committed to driving positive change within the chemical industry. She actively seeks out environmentally friendly solutions and promotes the adoption of sustainable practices. Akshita believes that a balance between economic growth and ecological responsibility is crucial for the industry's long-term success. She is dedicated to finding innovative ways to minimize environmental impact while maximizing efficiency and profitability.