Uses of Ferrous Sulphate Heptahydrate Explained
Why Ferrous Sulphate Heptahydrate Is Essential in Multiple Industries?

- May 27, 2025
- By Akshita Patel
Ever come across a bluish-green crystalline powder in a lab or industrial setup and wondered what it does? Chances are, you were looking at Ferrous Sulphate Heptahydrate—a compound with a long name but a wide range of practical applications.
You might be thinking, “Isn’t that just another chemical?”
Not quite.
Ferrous Sulphate Heptahydrate (FeSO₄·7H₂O) plays a critical role in industries like pharmaceuticals, water treatment, and even animal nutrition. From treating iron deficiency in humans and animals to helping purify water, this compound quietly works behind the scenes to keep key systems running smoothly
In this blog, we’ll break down:
- What exactly is Ferrous Sulphate Heptahydrate is,
- How it’s made and why it matters,
- Where it’s used, and
- What to keep in mind when storing or handling it.
If you work in health, water management, or manufacturing—or just want to learn more about this unsung industrial hero—you’re in the right place.
Let’s get started.
What is Ferrous Sulphate Heptahydrate?
Ferrous Sulphate Heptahydrate is an inorganic compound with the chemical formula FeSO₄·7H₂O. It is the hydrated form of ferrous sulfate, meaning it contains seven molecules of water bound to each molecule of iron(II) sulfate. This compound is one of the most common iron salts used in industrial and pharmaceutical applications.
Chemical Composition and Formula:
Formula: FeSO₄·7H₂O – It consists of ferrous (Fe²⁺) ions, sulfate (SO₄²⁻) ions, and water molecules.
The heptahydrate form ensures better solubility and easier handling in many applications compared to the anhydrous variant.
Physical Appearance: Ferrous Sulphate Heptahydrate typically appears as bluish-green crystals. The color is due to the presence of iron(II) ions combined with the water of crystallization. When exposed to air, it can slowly oxidize and turn yellow-brown as it converts to ferric sulfate (Fe³⁺).
Solubility and Stability: This compound is highly soluble in water, making it suitable for liquid formulations and applications requiring fast iron absorption. However, it is unstable when exposed to air and moisture for prolonged periods, as it can oxidize and lose its water of hydration, forming less effective or inactive compounds. Proper storage in airtight containers is essential to maintain its quality.
Common Forms: Ferrous Sulphate Heptahydrate is available in several physical forms depending on the intended use:
Crystalline powder – commonly used in pharmaceuticals and laboratory settings for precise dosing.
Granules – often used in bulk applications due to ease of handling and reduced dust generation.
This combination of solubility, availability, and versatility makes Ferrous Sulphate Heptahydrate a widely preferred source of iron in multiple non-agricultural industries.
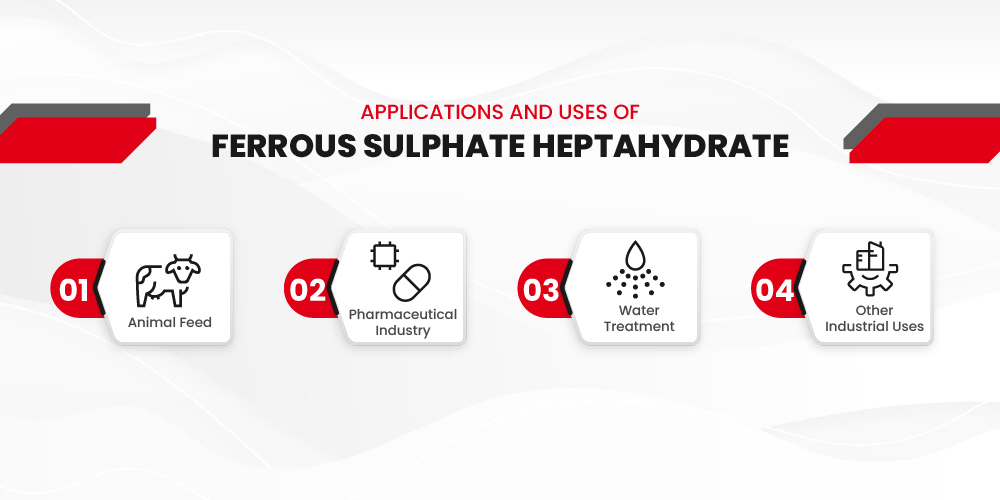
Applications and Uses of Ferrous Sulphate Heptahydrate
Ferrous Sulphate Heptahydrate is a multi-functional compound with broad utility across various non-agricultural sectors. Its rich iron content, high solubility, and reducing properties make it an essential ingredient in multiple industrial and commercial applications.
Animal Feed:
Iron is a critical trace element required for several physiological functions in animals. Ferrous Sulphate Heptahydrate is commonly used as an iron supplement in animal feed formulations, particularly for young or rapidly growing animals. Key functions in animal nutrition include:
- Hemoglobin synthesis: Iron is a fundamental component of hemoglobin, essential for oxygen transport in the blood. A deficiency can result in hypochromic, microcytic anemia.
- Enzyme activation: Iron-dependent enzymes play a key role in energy metabolism and immune response.
- Growth and reproduction: Adequate iron levels ensure healthy tissue development and fertility in breeding animals.
Use in feed production:
- Added in premixes, mineral licks, or fortified feed formulations.
- Particularly crucial in piglets, which are born with low iron reserves and require supplementation within days after birth.
Benefits of using Ferrous Sulphate Heptahydrate:
- Cost-effective compared to organic iron sources.
- High bioavailability when properly formulated.
- Easy to blend into feed due to its crystalline or granular forms.
Pharmaceutical Industry: Treating Iron Deficiency in Humans:
Ferrous Sulphate Heptahydrate is a first-line treatment for iron deficiency anemia—a condition affecting millions globally due to poor diet, blood loss, or chronic illness.
Key pharmaceutical uses:
Found in oral iron formulations like tablets, capsules, powders, and syrups.
Administered to treat:
- Iron-deficiency anemia
- Pregnancy-related anemia
- Post-surgical blood loss
- Fatigue and pallor associated with low hemoglobin levels
Bioavailability and Dosage Considerations:
Ferrous Sulphate Heptahydrate provides elemental iron, which is absorbed in the duodenum and upper jejunum. Bioavailability may vary depending on stomach pH, food intake, and presence of absorption enhancers like ascorbic acid (vitamin C). Overdose or improper usage can lead to side effects such as constipation, nausea, or iron overload, especially in individuals with conditions like hemochromatosis.
Pharmaceutical-grade requirements:
Must meet pharmacopeial standards (e.g., USP, BP, or IP) for purity, solubility, and iron content.
Manufacturing requires controlled environments to prevent oxidation and maintain stability.
Water Treatment: Purifying Water Through Coagulation and Precipitation
Ferrous Sulphate Heptahydrate is a widely used coagulant and flocculant in water and wastewater treatment processes. Its role is crucial in removing suspended solids, phosphates, and other pollutants. Key applications in water treatment:
- Flocculation and coagulation: When added to water, it reacts with dissolved oxygen and forms ferric hydroxide flocs, which trap fine particles and settle out as sludge.
- Phosphate removal: Forms insoluble iron phosphate, effectively reducing phosphate levels in effluent streams, especially in municipal sewage treatment plants, helping combat eutrophication in receiving water bodies.
- Color and odor removal: Helps eliminate hydrogen sulfide (rotten egg smell) and tannins in groundwater.
Advantages:
- Effective over a wide pH range
- Cost-efficient alternative to aluminum-based coagulants
- Less corrosive than ferric chloride, making it easier to handle
- Industries using ferrous sulfate for water treatment include:
- Municipalities (sewage treatment plants)
- Textile and dye industries
- Paper and pulp manufacturing
- Food processing plants
Other Industrial Uses: A Reliable Iron Source and Reducing Agent
Beyond feed, pharma, and water, Ferrous Sulphate Heptahydrate plays a supporting role in several chemical and industrial manufacturing processes:
- Reducing Agent in Chemical Processes:
- Ferrous sulfate acts as a reducing agent in redox reactions, converting more oxidized species (like hexavalent chromium) into their reduced, less toxic forms.
- Used in the cement industry for reducing chromium(VI) to chromium(III), which is significantly less hazardous.
- Manufacturing of Other Iron Compounds: Used as a precursor to synthesize compounds such as:
- Ferric sulfate – another coagulant for water treatment
- Iron oxide pigments – used in paints, coatings, and construction materials
- Iron-based catalysts – for chemical synthesis and petroleum refining
- Electroplating and Surface Treatment: Employed in some electroplating baths to provide a controlled supply of iron ions. Used in pickling solutions to remove rust or scale from metal surfaces before further processing.
- Soil Remediation and Environmental Cleanup: In contaminated industrial zones, ferrous sulfate is used to immobilize heavy metals or neutralize toxic substances in the soil through redox reactions.
The widespread utility of Ferrous Sulphate Heptahydrate across animal nutrition, pharmaceuticals, water treatment, and industrial chemistry speaks volumes about its importance. Its effectiveness, cost-efficiency, and multi-functional nature make it an indispensable compound in several sectors that prioritize health, safety, and environmental responsibility.
Safety and Handling Guidelines
While Ferrous Sulphate Heptahydrate is widely used across industries, proper safety and handling practices are essential to prevent health hazards and ensure safe storage and usage. This section outlines the key considerations regarding its toxicity, handling protocols, storage recommendations, and emergency measures.
Toxicity Levels: While generally safe in regulated amounts, excessive ingestion can lead to iron poisoning, and inhalation of dust may irritate the respiratory tract. Skin and eye contact can cause mild irritation.
Handling Precautions
- Wear gloves, goggles, and a mask when handling.
- Work in well-ventilated areas to minimize dust exposure.
- Wash hands thoroughly after use and avoid eating or drinking near the material.
Storage Conditions
- Store in a cool, dry, airtight container to prevent moisture absorption and caking.
- Keep away from acids, alkalis, and oxidizing agents.
- Avoid heat and direct sunlight.
First Aid Measures
- Inhalation: Move to fresh air; seek help if breathing is difficult.
- Skin/Eyes: Rinse with water for several minutes; remove contaminated clothing.
- Ingestion: Do not induce vomiting; seek medical attention immediately.
Ferrous Sulphate Heptahydrate plays a crucial role across multiple industries—from animal nutrition and pharmaceuticals to water treatment and chemical manufacturing. Its effectiveness, versatility, and wide availability make it an essential compound in various industrial processes. However, like any chemical substance, it must be handled and stored with proper care to ensure safety and maintain quality.
At Annexe Chem Pvt. Ltd., we pride ourselves on being a leading manufacturer of high-purity Ferrous Sulphate Heptahydrate, delivering consistent quality to meet the diverse needs of our clients. With a commitment to excellence and industry standards, we support businesses with reliable and efficient chemical solutions.
For inquiries or bulk orders, get in touch with our team today.

Akshita Patel
As an advocate for sustainability, Akshita is committed to driving positive change within the chemical industry. She actively seeks out environmentally friendly solutions and promotes the adoption of sustainable practices. Akshita believes that a balance between economic growth and ecological responsibility is crucial for the industry's long-term success. She is dedicated to finding innovative ways to minimize environmental impact while maximizing efficiency and profitability.
Related Blogs

- April 9, 2024
- By Akshita Patel
The Comprehensive Guide to Sodium Dihydrogen.
At its core, Sodium Dihydrogen Phosphate Dihydrate, with its chemical formula NaH2PO4.2H2O, embodies a symphony of.

- March 19, 2024
- By Akshita Patel
From Sunscreens to Sustainable Solutions: Exploring.
Zinc Oxide stands tall as a versatile and indispensable compound. From its remarkable properties to its.