Calcium Sulphate Dihydrate: Top Industrial Uses
What Is Calcium Sulphate Dihydrate? Key Applications Across Industries

- May 5, 2025
- By Akshita Patel
Ever wondered what holds your walls together, helps doctors make casts, and even shapes stunning sculptures? The answer lies in a seemingly humble mineral—Calcium Sulphate Dihydrate, also known as gypsum. While it may not have a flashy name, this compound is a quiet game-changer in construction, medicine, education, and the arts.
From drywall panels and dental molds to classroom chalk and statues, Calcium Sulphate Dihydrate shows up in more places than you’d expect. In this blog, we’ll break down what it is, why it matters, and how it’s used across a range of industries you probably interact with every day.
What is Calcium Sulphate Dihydrate?
Calcium Sulphate Dihydrate (CaSO₄·2H₂O) is a naturally occurring mineral compound composed of calcium, sulfur, oxygen, and water. It’s most commonly known in its natural form as gypsum, and when heated to remove water content, it transforms into Plaster of Paris—a widely used material across various sectors. Despite its simple chemical structure, Calcium sulfate dihydrate plays a surprisingly vital role in modern life. It is an essential component in the construction industry, where it’s used in products like drywall and plaster. In medical fields, it’s valued for making orthopedic casts and dental molds. It even finds a place in educational tools and artistic creations, helping to shape everything from classroom models to intricate sculptures.
Key Properties Of Calcium Sulphate Dihydrate
- Physical Properties: Calcium Sulphate Dihydrate is typically white to gray in color, with a fine, powdery or crystalline texture. It has a Mohs hardness of around 2, making it soft and easy to scratch or mold. It’s also lightweight and has a relatively low density, which makes it ideal for construction and decorative applications.
- Chemical Properties: Chemically stable under normal conditions, it doesn’t react easily with most substances. However, it decomposes when heated, losing its water content and converting into Plaster of Paris (CaSO₄·½H₂O). This property is central to its utility in mold-making and construction.
- Hygroscopic Nature: Calcium Sulphate Dihydrate has low hygroscopicity, meaning it doesn’t easily absorb moisture from the air. This makes it suitable for environments where moisture control is important. However, in its anhydrous or hemihydrate forms, it can absorb water readily, allowing it to set and harden, which is crucial in plastering and casting.
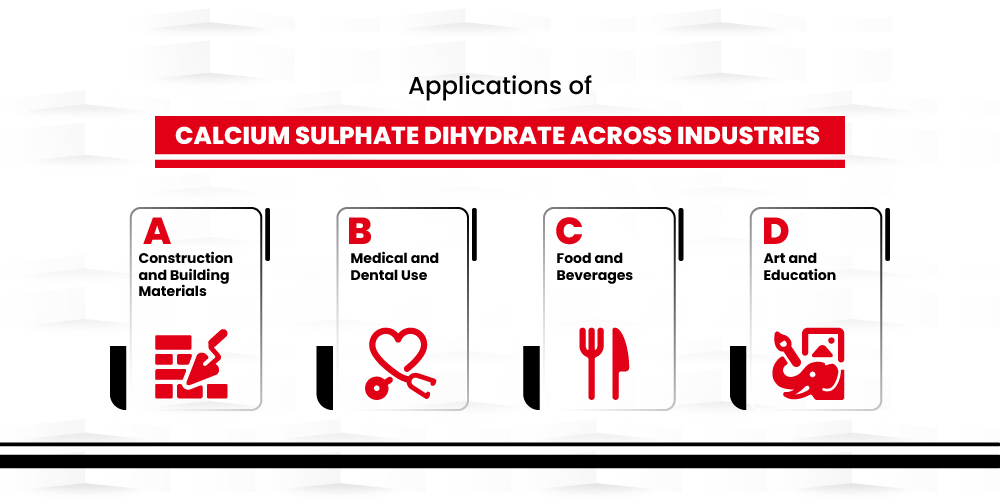
Applications of Calcium Sulphate Dihydrate Across Industries
Construction and Building Materials:
One of the most widespread uses of Calcium Sulphate Dihydrate is in the construction sector, where it is prized for its adaptability, affordability, and performance-enhancing qualities.
- Drywall (Gypsum Board): The core of drywall panels is made from compressed Calcium Sulphate Dihydrate. These boards are extensively used for wall and ceiling construction in residential, commercial, and institutional buildings. They offer sound insulation, quick installation, and cost-effective coverage, making them a staple in modern construction.
- Plaster and Plaster of Paris: When gypsum is calcined (heated to around 150°C), it forms Calcium Sulphate Hemihydrate—commonly known as Plaster of Paris. When mixed with water, it rehydrates and hardens rapidly. This makes it perfect for smooth wall finishes, decorative cornices, and ceiling work.
- Cement Additive: In cement manufacturing, gypsum is added during the grinding process to regulate setting time. Without it, cement would set too quickly, making it difficult to work with on construction sites.
Key benefits in construction:
- Fire resistance: Gypsum contains around 21% chemically bound water. When exposed to fire, this water vaporizes, slowing down heat transfer and delaying structural failure. For this reason, gypsum boards are often used in fire-rated wall assemblies.
- Workability: Gypsum plaster is easy to mix, spread, and mold. It offers a smooth finish with minimal shrinkage or cracking, reducing post-installation defects.
- Environmentally friendly: Gypsum is non-toxic, recyclable, and contributes to indoor air quality by regulating humidity.
Medical and Dental Use:
Calcium Sulphate Dihydrate plays a critical role in healthcare, especially in orthopedics, dentistry, and surgical applications.
- Orthopedic Casting: Plaster of Paris is used to make orthopedic casts that immobilize broken bones. It’s preferred because it molds easily when wet, sets quickly, and forms a firm but breathable shell around the injury site.
- Bone Grafting: In surgical procedures, synthetic bone grafts made from medical-grade Calcium Sulphate Dihydrate are used as scaffolds for bone regeneration. These grafts are biocompatible and resorbable, meaning they gradually dissolve as new bone tissue forms.
- Dental Applications: Dentists use gypsum-based materials to make accurate molds of patients’ teeth for crowns, bridges, and orthodontic devices. Its fine detail reproduction and dimensional stability make it ideal for this purpose.
- Surgical Molds and Splints: Surgeons and prosthetic designers often rely on gypsum for making molds of limbs or other body parts for prosthetics or treatment planning.
Its non-toxic, inexpensive, and easy-to-use nature makes it an indispensable material in medical practice worldwide.
Food and Beverages:
Calcium Sulphate Dihydrate is also recognized by food safety authorities as a safe additive, known by its code E516. Though it’s used in relatively small quantities, its impact is significant in food processing and beverage production.
- Tofu Production: In the tofu-making process, Calcium Sulphate acts as a coagulant. When added to hot soy milk, it reacts with proteins to form curds, resulting in a firm yet smooth texture. It’s particularly valued for producing traditional Chinese-style tofu.
- Baking Industry: It is occasionally used as a dough conditioner or flour treatment agent. It helps regulate acidity and improve texture.
- Brewing: In beer brewing, Calcium Sulphate (gypsum) is added to the water to enhance mineral content. This process, known as “water profiling,” helps achieve specific flavor profiles and improve beer clarity by promoting yeast flocculation and protein precipitation.
Its neutral taste, non-reactive nature, and GRAS (Generally Recognized As Safe) status make it a valuable component in food technology.
Art and Education:
In the worlds of art and education, Calcium Sulphate Dihydrate continues to be a go-to material for creative expression and academic learning.
- Artistic Sculptures and Casts: Artists use Plaster of Paris to create statues, reliefs, and molds because it captures fine details and sets quickly. Once dried, it can be carved, painted, or finished in various ways. It’s widely used in museums and restoration projects.
- Mold-Making: In both commercial and academic settings, gypsum molds are used for ceramics, jewelry design, and model-making. The material is affordable and replicates complex forms with high precision.
- Educational Models: Gypsum-based materials are often used to produce anatomy models, planetary surfaces, volcanoes, and other classroom teaching aids. Their low cost and ease of use make them ideal for schools and colleges.
- Theatrical Props and Set Design: Due to its lightweight and quick-setting properties, it’s frequently used in set design for movies, theater productions, and exhibitions.
Whether in a studio, classroom, or lab, the versatility of Calcium Sulphate Dihydrate makes it a creative enabler and teaching tool across generations.
Health and Safety Considerations
While Calcium Sulphate Dihydrate is generally considered non-toxic and safe for industrial and household use, appropriate handling practices are essential to avoid potential health risks.
Inhalation and Skin Contact Precautions: Inhalation of gypsum dust during mixing, sanding, or grinding can irritate the respiratory tract. Prolonged exposure may lead to coughing or throat discomfort. Workers should wear masks or respirators in dusty environments.
Skin contact may cause dryness or irritation, especially with prolonged exposure. Though not highly reactive, wearing gloves and protective clothing is recommended when handling large quantities.
Eye contact with dust or slurry can cause irritation; safety goggles should be used in active work areas.
Safe Handling and Storage
- Store in dry, well-ventilated areas to prevent premature hydration or clumping.
- Use sealed containers or bags to limit exposure to moisture and reduce dust dispersion.
- Avoid storing near incompatible materials like strong acids, which can lead to unwanted reactions.
Calcium Sulphate Dihydrate, commonly known as gypsum, is far more than just a building material. Its versatility spans across construction, healthcare, food production, and the arts—offering unique benefits in each sector. With properties like fire resistance, moldability, and chemical stability, it continues to be an essential material in both industrial and everyday applications.
However, like any widely used resource, it comes with responsibilities. Safe handling practices, proper disposal, and mindful sourcing are critical to ensuring minimal health and environmental impact. As industries move toward more sustainable practices, understanding the full scope of this mineral’s applications and impacts helps us use it wisely and efficiently.
At the forefront of supplying high-quality chemical compounds like Calcium Sulphate Dihydrate is Annexe Chem Pvt Ltd—a trusted name in the chemical manufacturing industry. With a strong commitment to quality, safety, and customer satisfaction, Annexe Chem continues to support a wide range of industries with reliable and sustainable chemical solutions.
Whether you’re looking for industrial-grade materials or custom chemical formulations, Annexe Chem Pvt Ltd delivers excellence you can depend on.

Akshita Patel
As an advocate for sustainability, Akshita is committed to driving positive change within the chemical industry. She actively seeks out environmentally friendly solutions and promotes the adoption of sustainable practices. Akshita believes that a balance between economic growth and ecological responsibility is crucial for the industry's long-term success. She is dedicated to finding innovative ways to minimize environmental impact while maximizing efficiency and profitability.
Related Blogs

- April 7, 2025
- By Akshita Patel
Potassium Dihydrogen Phosphate: A Versatile Compound.
When you think of essential compounds in food, medicine, and industry, Potassium Dihydrogen Phosphate (KH₂PO₄) might.

- March 19, 2024
- By Akshita Patel
From Sunscreens to Sustainable Solutions: Exploring.
Zinc Oxide stands tall as a versatile and indispensable compound. From its remarkable properties to its.