What is Sodium Hydroxide Powder? Know About Its Applications and Safety Precautions - Annexe Chem Pvt Ltd
What is Sodium Hydroxide Powder? Know About Its Applications and Safety Precautions

- November 18, 2024
- By Akshita Patel
Imagine a powder so powerful it can unclog drains, create soap, purify water, and even help make paper. Meet sodium hydroxide powder, a seemingly humble white substance with transformative abilities. Known as caustic soda, this versatile compound is a staple in industries and households alike, yet it demands respect due to its potent, corrosive properties. Whether you’re a professional chemist or a DIY enthusiast, understanding sodium hydroxide’s fascinating uses and handling it safely can unlock a world of possibilities. Let’s dive into the remarkable world of sodium hydroxide powder and uncover how its magic works in everyday life.
What is Sodium Hydroxide Powder?
Sodium hydroxide powder, commonly known as caustic soda or lye, is a strong alkaline compound with the chemical formula NaOH. It is composed of sodium (Na), oxygen (O), and hydrogen (H), forming an ionic bond that gives it a high reactivity, especially with water and acids. Sodium hydroxide is highly effective in breaking down organic materials and neutralizing acids, making it an essential component in many industrial and cleaning applications.
Key Characteristics of Sodium Hydroxide Powder:
- Appearance: Sodium hydroxide powder is a white, crystalline, and odorless substance.
- Hygroscopic Nature: It is highly hygroscopic, meaning it readily absorbs moisture from the air, which can cause it to clump or form a solution when exposed to humidity.
- Corrosive Properties: Sodium hydroxide is highly caustic and corrosive, capable of breaking down organic matter, which is why it is used in drain cleaners and industrial cleaners.
Key Applications of Sodium Hydroxide Powder
Sodium hydroxide powder plays a significant role in various fields due to its strong alkaline properties and effectiveness as a chemical reactant. Here’s a closer look at its primary applications in industrial, household, and laboratory settings:
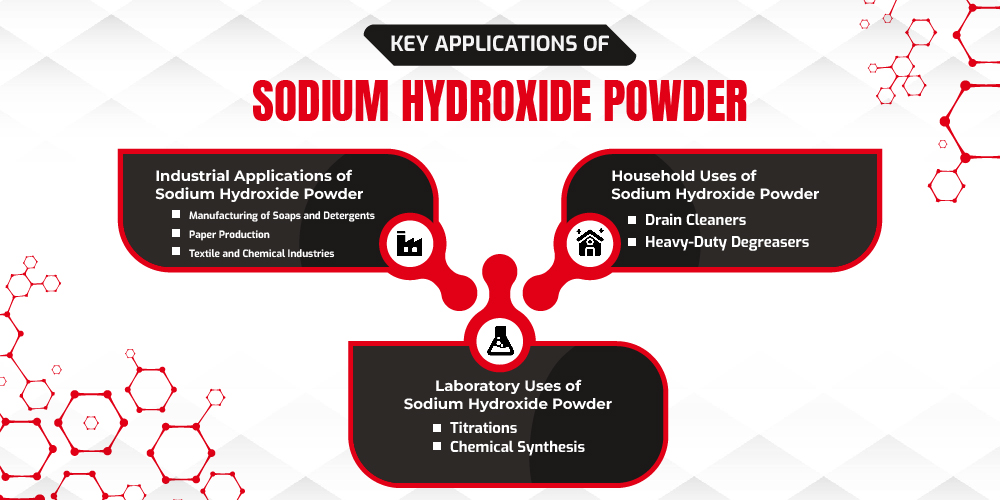
Industrial Applications of Sodium Hydroxide Powder
- Manufacturing of Soaps and Detergents: Sodium hydroxide is a key ingredient in the production of soaps and detergents. It reacts with animal fats and vegetable oils in a process called saponification, resulting in soap. This reaction breaks down fats and oils into fatty acid salts (soap) and glycerin. In detergent production, sodium hydroxide helps remove grease, dirt, and stains. Its ability to cut through oils makes it invaluable for formulating products that deliver deep-cleaning power.
- Paper Production: The pulp and paper industry relies heavily on sodium hydroxide for the kraft process, a method of producing paper pulp from wood chips. Here, sodium hydroxide helps dissolve lignin, the compound that binds wood fibers together, making it easier to extract cellulose fibers for paper-making. Additionally, sodium hydroxide is used to bleach paper pulp, contributing to brighter and whiter paper products.
- Textile and Chemical Industries: Sodium hydroxide is used in textile processing, particularly for mercerizing cotton, a process that strengthens fibers, enhances dye uptake, and adds a lustrous finish. It is also used for cleaning fabrics by removing impurities and natural oils.
Sodium hydroxide acts as a pH control agent and a neutralizer in various chemical reactions, enabling precision and efficiency. In the production of synthetic fibers, plastics, pharmaceuticals, and other chemical compounds, sodium hydroxide’s reactivity with acidic substances plays a crucial role.
Household Uses of Sodium Hydroxide Powder
- Drain Cleaners: Sodium hydroxide is a popular ingredient in drain cleaners because of its ability to break down organic materials such as hair, grease, and food residue that commonly clog drains. When combined with water, it generates heat and produces a strong, caustic solution that dissolves blockages effectively. As a result, sodium hydroxide-based drain cleaners are among the most effective solutions for unclogging household pipes.
- Heavy-Duty Degreasers: Sodium hydroxide’s powerful grease-cutting ability makes it ideal for heavy-duty cleaning tasks, particularly in kitchens and industrial settings. It’s often found in degreasing products designed for ovens, grills, and other surfaces that accumulate tough, baked-on grease. By breaking down stubborn oils and residues, sodium hydroxide provides a thorough clean, restoring surfaces to their original state.
Laboratory Uses of Sodium Hydroxide Powder
- Titrations: Sodium hydroxide is commonly used in acid-base titrations, a technique in chemistry to determine the concentration of an unknown acid. Since it is a strong base with a predictable reactivity, it serves as a reliable titrant for neutralizing acids and determining their concentration. Its high reactivity and ease of measurement make it ideal for analytical chemistry experiments, as it provides accurate and reproducible results.
- Chemical Synthesis: In laboratories, sodium hydroxide serves as a crucial reagent in various chemical synthesis processes, enabling the production of organic compounds, pharmaceuticals, and other chemicals. It participates in nucleophilic substitution reactions, where it helps break down complex molecules into simpler components. This versatility makes sodium hydroxide indispensable in synthetic chemistry, where precise reactions are needed to achieve desired products.
Sodium hydroxide powder is a powerhouse ingredient across these diverse applications, demonstrating its essential role in both industrial production and everyday household tasks. Its ability to dissolve, neutralize, and catalyze reactions makes it an irreplaceable tool in numerous fields.
Safety Precautions While Handling Sodium Hydroxide Powder
Sodium hydroxide powder, while versatile and useful, is also highly caustic and can pose significant hazards if not handled properly. Its strong corrosive properties make it crucial to follow strict safety precautions to prevent injuries and accidents. Below are essential guidelines to ensure safe handling, storage, and emergency response when working with sodium hydroxide powder.
Potential Hazards Of Sodium Hydroxide Powder
- Corrosive Nature: Sodium hydroxide is extremely corrosive, meaning it can cause severe burns on contact with skin or other organic materials. Its ability to break down organic substances is what makes it useful in applications like drain cleaning but also hazardous when mishandled.
- Skin and Eye Irritation: Exposure to sodium hydroxide powder can lead to intense skin and eye irritation, causing redness, pain, and potential chemical burns. If inhaled, it can irritate the respiratory system and may lead to coughing, difficulty breathing, or even damage to lung tissue in severe cases.
Protective Measures While Handling Sodium Hydroxide Powder
Personal Protective Equipment (PPE)
- Gloves: Wear chemical-resistant gloves to prevent skin contact. Nitrile or rubber gloves are generally recommended for their durability against caustic substances.
- Goggles: Use safety goggles or a face shield to protect your eyes from accidental splashes or airborne particles.
- Protective Clothing: Wear long sleeves, lab coats, and aprons to protect your skin and clothing from spills and accidental contact.
Storage Requirements
- Airtight Containers: Sodium hydroxide powder is highly hygroscopic, meaning it absorbs moisture from the air. To prevent clumping and unintended reactions, store it in airtight, corrosion-resistant containers that are properly labeled.
- Cool, Dry Place: Store sodium hydroxide powder in a cool, dry environment, away from direct sunlight and heat sources. Exposure to humidity or water can lead to an exothermic reaction (releasing heat) and potentially harmful splashes or spills.
- Segregate from Incompatible Substances: Keep sodium hydroxide separate from acids, metals, and organic compounds, as reactions with these substances can be dangerous and produce hazardous by-products.
Work in a Well-Ventilated Area: When working with sodium hydroxide powder, ensure that the workspace is well-ventilated. Using it in an enclosed or poorly ventilated area increases the risk of inhaling airborne particles or fumes, especially if the powder reacts with water.
Sodium hydroxide powder may look like a simple, white substance, but its powerful properties make it invaluable across industries, households, and laboratories. From creating everyday products like soap and paper to unclogging drains and aiding in complex chemical synthesis, sodium hydroxide is a crucial tool with a far-reaching impact. However, its strength also calls for respect and caution—understanding its hazards, handling it properly, and knowing emergency procedures are essential steps to harnessing its benefits safely. Whether you’re a professional or a DIY enthusiast, handling sodium hydroxide responsibly allows you to tap into its vast potential while prioritizing safety.

Akshita Patel
As an advocate for sustainability, Akshita is committed to driving positive change within the chemical industry. She actively seeks out environmentally friendly solutions and promotes the adoption of sustainable practices. Akshita believes that a balance between economic growth and ecological responsibility is crucial for the industry's long-term success. She is dedicated to finding innovative ways to minimize environmental impact while maximizing efficiency and profitability.
Related Blogs

- January 24, 2024
- By Akshita Patel
Thiourea: Unraveling Its Diverse Applications
Thiourea is an organosulfur compound with the formula SC(NH₂)₂ and the structure H₂N−C−NH₂. The sulfur atom.

- June 4, 2024
- By Akshita Patel
The Importance of Di Sodium Hydrogen.
Chemical compounds are the building blocks of our modern world, playing crucial roles in a wide.


 Meet Us at Chem Expo 2025 – Hall 2, Stall 2D29A | April 2025
Meet Us at Chem Expo 2025 – Hall 2, Stall 2D29A | April 2025