The Complete Guide to Sodium Chloride: Uses, Myths, and Health Implications - Annexe Chem Pvt Ltd
The Complete Guide to Sodium Chloride: Uses, Myths, and Health Implications

- June 25, 2024
- By Akshita Patel
Did you know that the average human body contains about 250 grams of sodium chloride, roughly the same amount as a box of table salt? This simple yet essential compound, commonly known as salt, is a cornerstone of human civilization, playing a critical role in everything from culinary delights to industrial processes. Whether sprinkled on your dinner or used to melt icy roads, sodium chloride is more than just a seasoning – it’s a fundamental element of life as we know it.
What is Sodium Chloride?
Sodium chloride is a chemical compound with the formula NaCl, consisting of one sodium (Na) ion and one chloride (Cl) ion. It is an ionic compound, meaning it is formed by the electrostatic attraction between positively charged sodium ions and negatively charged chloride ions. This crystalline structure gives sodium chloride its characteristic solid, granular appearance and makes it highly soluble in water.
Historical Background and Discovery
Sodium chloride has a rich history that dates back to ancient civilizations. Its significance is evident in its historical role as a valuable trade commodity and a critical preservative.
- Ancient Civilizations: Egyptians used salt for preservation by 6,000 BC. The Chinese documented salt production by 2,700 BC. Romans paid soldiers with salt, coining “salary.”
- Medieval Period: In the Middle Ages, salt influenced trade and economics, with Venice gaining power through its control.
- Modern Discovery: In the 19th century, Sir Humphry Davy confirmed sodium chloride’s composition using electrolysis.
Sodium chloride’s journey from a precious ancient commodity to an everyday kitchen staple underscores its enduring importance and versatility. As we continue to explore its uses and benefits, it’s clear that this humble compound is much more than just a seasoning – it’s a fundamental building block of our world.
Uses of Sodium Chloride
Sodium chloride, or common table salt, is a versatile compound with a wide range of applications in culinary, industrial, and medical fields. Let’s explore the various ways in which this essential substance is utilized.
Culinary Uses of Sodium Chloride
Table Salt and Its Varieties: Sodium chloride is commonly recognized as table salt, an essential seasoning in kitchens worldwide. Various types of salt are available, each with unique characteristics:
- Table Salt: Refined and often iodized, making it suitable for everyday use.
- Kosher Salt: Coarse-grained and free of additives, preferred in professional kitchens.
- Sea Salt: Harvested from evaporated seawater, often less processed and containing trace minerals.
- Himalayan Pink Salt: Mined from ancient sea beds, known for its distinctive pink color and mineral content.
Importance of Sodium Chloride in Cooking and Baking
Salt plays a crucial role in cooking and baking:
- Flavor Enhancement: Salt enhances the natural flavors of food, making dishes more palatable.
- Preservation: Historically used to preserve meats and vegetables, salt inhibits the growth of bacteria.
- Baking: In baking, salt regulates yeast fermentation, strengthens gluten, and enhances texture and flavor.
- Brining: Brining meat in a salt solution helps retain moisture and improves tenderness.
Industrial Uses of Sodium Chloride
Chemical Industry
Sodium chloride is a fundamental raw material in the chemical industry:
- Chlorine and Sodium Hydroxide Production: Through electrolysis, sodium chloride is separated into chlorine gas and sodium hydroxide (caustic soda), essential for manufacturing plastics, paper, detergents, and more.
- Soda Ash Production: Used in the production of sodium carbonate, crucial for glass, soap, and paper industries.
Water Softening
In water softening systems, sodium chloride regenerates ion exchange resins:
- Ion Exchange Process: Sodium ions replace calcium and magnesium ions in hard water, preventing scale buildup in pipes and appliances, and improving soap efficiency.
De-icing Roads
Sodium chloride is extensively used for de-icing and preventing icy roads:
- Melting Ice: Salt lowers the freezing point of water, effectively melting ice and snow on roadways.
- Safety: Ensures safer driving conditions during winter, reducing the risk of accidents.
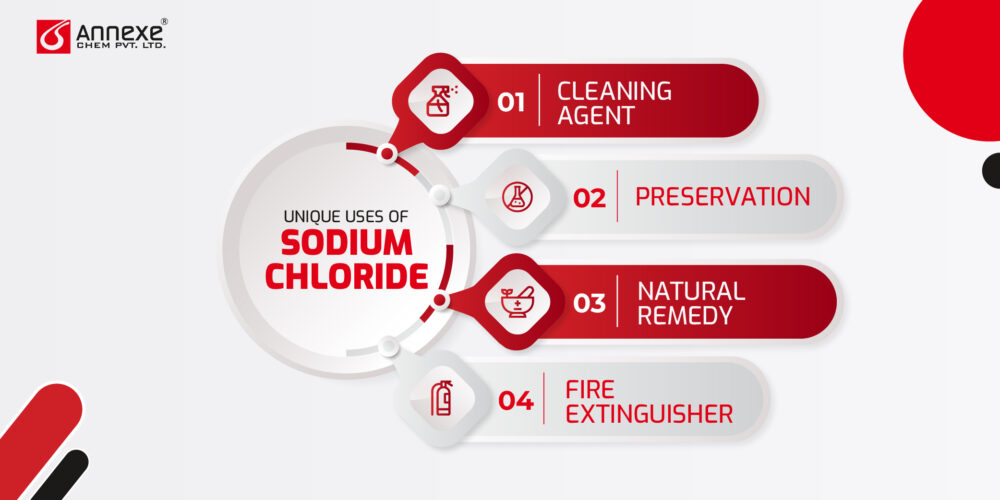
Health and Medical Uses of Sodium Chloride
Electrolyte Balance in the Body
Sodium chloride is vital for maintaining proper bodily functions:
- Hydration: Sodium and chloride ions help regulate fluid balance, nerve function, and muscle contractions.
- Electrolyte Solutions: Sports drinks and oral rehydration solutions contain sodium chloride to restore electrolytes lost through sweat and dehydration.
Medical Treatments
Sodium chloride solutions are essential in medical treatments:
- Saline Solutions: Used for intravenous infusions to rehydrate patients, deliver medications, and maintain blood pressure during surgery.
- Wound Care: Saline is used to clean wounds and prevent infections.
- Nasal Irrigation: Saline nasal sprays relieve nasal congestion and sinus issues.
Sodium chloride’s versatility and essential properties make it indispensable in daily life, from enhancing our meals to supporting industrial processes and medical treatments. Understanding its diverse uses highlights the importance of this seemingly simple compound in maintaining modern society’s functionality and health.
Health Implications of Sodium Chloride
Sodium chloride, while essential for bodily functions, must be consumed in appropriate amounts to avoid health risks. This section explores the dietary role of sodium chloride, its sources, potential health risks, and benefits.
Sodium Chloride in Diet
Health organizations provide guidelines on the recommended daily intake of sodium:
- Adults: The World Health Organization (WHO) recommends an intake of less than 2,000 mg of sodium per day, roughly equivalent to 5 grams (about one teaspoon) of salt.
- Children: The recommended intake for children varies by age, generally lower than for adults to match their dietary needs and body size.
Sources in Food
Sodium chloride is present in various foods, both naturally and through processing:
- Natural Sources: Vegetables, dairy products, and meats contain natural sodium, though in relatively low amounts.
- Processed Foods: A significant source of dietary sodium comes from processed and packaged foods such as:
- Canned soups and vegetables: Often high in added salt for preservation and flavor.
- Snack foods: Chips, crackers, and pretzels are typically high in sodium.
- Prepared meals: Frozen dinners, pizzas, and fast foods contain substantial amounts of added salt.
- Condiments: Soy sauce, ketchup, and salad dressings are common high-sodium culprits.
Health Risks of Excess Sodium
High Blood Pressure (Hypertension):
- Mechanism: High sodium levels cause the body to retain water, increasing blood volume and pressure on blood vessel walls.
- Prevalence: Hypertension affects millions globally and is a significant risk factor for heart disease and stroke.
Cardiovascular Diseases:
- Heart Disease: Elevated blood pressure strains the heart, leading to conditions such as heart attacks and heart failure.
- Stroke: Increased pressure and damage to blood vessels heighten the risk of strokes.
Health Benefits- Necessary for Bodily Functions
Sodium chloride is crucial for various physiological functions:
- Electrolyte Balance: Maintains proper fluid balance within cells and blood vessels.
- Nerve Function: Essential for transmitting nerve impulses.
- Muscle Contraction: Helps muscles contract and relax, including the heart muscle.
Prevention of Iodine Deficiency
- Iodine Supplementation: Iodine is an essential mineral for thyroid function and hormone production.
- Deficiency Prevention: Iodized salt prevents iodine deficiency disorders such as goiter and hypothyroidism, particularly in regions where natural iodine levels in food and water are low.
Sodium chloride, while essential in moderation, poses health risks when consumed excessively. Understanding its sources in our diet and adhering to recommended intake guidelines can help mitigate these risks. Moreover, iodized salt remains a crucial public health measure to prevent iodine deficiency. Balancing sodium chloride intake is key to maintaining good health and preventing chronic diseases.

Interesting Facts and Myths of Sodium Chloride
Sodium chloride, or table salt, is surrounded by various myths and fascinating facts that highlight its unique characteristics and multifaceted uses. Let’s explore some common misconceptions and uncover intriguing details about this ubiquitous compound.
Myth 1: Sea Salt is Healthier than Table Salt
Debunked: Despite popular belief, sea salt, and table salt contain similar amounts of sodium by weight. The main difference lies in their texture and trace minerals. While sea salt may contain small amounts of minerals like magnesium and calcium, these differences are not significant enough to impact health substantially. The key is moderation in consumption, regardless of the type.
Myth 2: Cutting Out Salt Completely is Beneficial
Debunked: Sodium is an essential nutrient required for vital bodily functions, including nerve impulse transmission, muscle contraction, and fluid balance. Eliminating salt can lead to hyponatremia, a dangerous condition caused by low sodium levels. It’s important to reduce excessive intake, not eliminate it.
Myth 3: Only Older Adults Need to Worry About Salt Intake
Debunked: High sodium intake affects people of all ages, not just older adults. Children and adolescents are also at risk of developing hypertension and cardiovascular diseases later in life due to high sodium consumption from processed foods. Everyone should be mindful of their salt intake to maintain long-term health.

Akshita Patel
As an advocate for sustainability, Akshita is committed to driving positive change within the chemical industry. She actively seeks out environmentally friendly solutions and promotes the adoption of sustainable practices. Akshita believes that a balance between economic growth and ecological responsibility is crucial for the industry's long-term success. She is dedicated to finding innovative ways to minimize environmental impact while maximizing efficiency and profitability.
Related Blogs

- June 18, 2024
- By Akshita Patel
Understanding Calcium Hydroxide: Properties, Uses, and.
Calcium Hydroxide, often known by its common names slaked lime or hydrated lime, is a versatile.

- December 18, 2023
- By Akshita Patel
Zinc Gluconate: A Comprehensive Guide
Zinc Gluconate is a chemical compound formed by combining zinc, an essential mineral, with gluconic acid,.


 Meet Us at Chem Expo 2025 – Hall 2, Stall 2D29A | April 2025
Meet Us at Chem Expo 2025 – Hall 2, Stall 2D29A | April 2025